How to Determine Which Layer Is Aqueous Using Kitchen
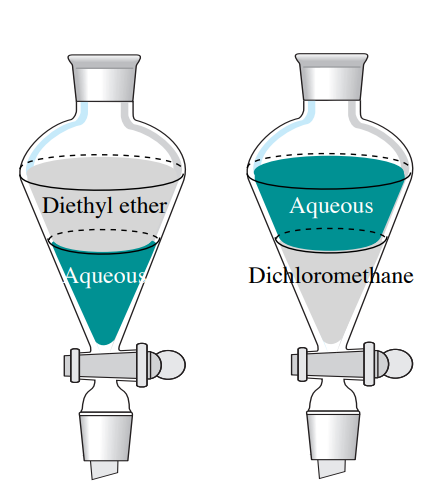
In the right separating funnel the aqueous layer is on the top meaning the organic layer must be more dense than water. These are the type of questions you would have to consider to answer your question.
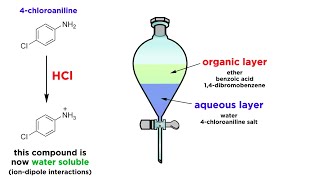
Separating Components Of A Mixture By Extraction Youtube
The lemon essential oil on the upper layer is removed.
. Get the most out of your space and your kitchen layout. Learn how to properly measure your kitchen and check out our design tips for different kitchen floor plans. Depend on the solubility of the compound you.
Most organic solvents will be less dense than water unless youre working with halogenated solvents like methylene chloride chloroform or carbon tetrachloride. Note that in almost every case one of the solvents is water or an aqueous solution. A practical layout option for small and large kitchens the L-shaped kitchen has cabinets along two perpendicular walls.
Ether is not soluble because theres not a strong enough dipole between the O and the C to give the O enough of a partial negative charge that would make it water soluble. We review their content and use your feedback to keep the quality high. However protein concentration determination in the protein-NPs has so far not been reported.
It is a common observation that when oil and water are poured into the same container they separate into two phases or layers because they are immiscible. Saturate the aqueous layer with sodium chloride and then extract either with chloroform or ethyl acetate. It is easier to isolate it from a small amount of solution than from the waste container.
Show activity on this post. In this Letter we present a simple and nondestructive approach to quantify the protein concentration in the protein-NPs aqueous solution using circular dichroism CD spectroscopy. It is important to note that the upper layer is that which is less dense.
The duration of the fragrance of the perfume last is tested. Each time you form a new aqueous layer with a new compound you can isolate it in a test tube. This answer is useful.
However in an ABS both. Look at the table on the previous slide. To answer this question you need to know about liquidliquid extraction acid-base chemistry and intermolecular interactions.
How many 100 mL chloroform extractions would be required to extract at least 90 of Y from a 500 mL aqueous solution of water. Isolate and save the bottom layer. To determine the aqueous layer- The NaHCO3 solution is an aqueous solution water is the solvent.
Who are the experts. The organic layer is always found at the top of the mixture while the aqueous layer is found at the bottom of the mixture. Without tasting smelling or removing either layer from the container how can you determine which layer is the aqueous layer using just material found in a typical kitchen.
The design of a kitchen is tied closely to the layout. This is because theres not a big enough electronegativity difference. Determine which layer is organic and which is aqueous.
While you can have the legs of. In a chemical equation the symbol aq follows a species name to indicate that it is in aqueous solution. Therefore if I use any piece of rod and an empty container found in the kitchen to perform a simple decantation the top layer that comes out first is.
4 points Calculations Answer 7 As you are cleaning out the shared refrigerator in the kitchen of your. 1-use measuring cylinder to measure how a set volume of the solution with known conc. HClaq NaOHaq NaClaq H2Ol HCl a q NaOH a q NaCl a q H 2 O l A strong acid and a weak base yield a weakly acidic solution not because of the strong acid involved but because of the conjugate acid of the weak base.
For example a mixture of tert-butyl methyl ether and water will separate into two layers with the ether layer density 074 being on top of the water layer density 10. Most common organic solvents possess a lower density than water. An aqueous solution is any solution in which water H 2 O is the solvent.
To perform this process an organic solvent ie. Add a small amount of water to the separatory funnel and closely watch to see which layer the newly added water joins. Water is more dense than ether and sinks to the bottom while the ether sits on top.
2-place out white tile with drawn cross 3-add a known volume of the other substance to that solution 4-record time taken for cross to dissapear out of view 5-record final temp 6-repeat using water baths or ice baths to results for different temps. 4 Compound X has a distribution coefficient K of 15 between diethyl ether and water indicating it is more soluble in diethyl ether. The ether layer is then removed and it is then evaporated off to leave just the.
U-Shape Kitchen Islands have widths that range from 15-18 46-55 m with overall depths and island dimensions that vary as needed. It would be appreciated if someone could help. A weak acid and a strong base yield a weakly basic solution.
This answer is not useful. If you are not sure which layer is organic and which one is aqueous take a small sample of both layers and add some water Which layer did increase in volume. The experiment is repeated from step 4 to step 6 using 15mL of 55 40 25 and 10 ethanol.
Since ether is less dense than water it will separate out on top of the aqueous layer. U-Shape Kitchen Islands should be planned with an overall area of roughly 155 ft2 144 m2. Updated on September 10 2019.
Kitchen Planning Guide. Experts are tested by Chegg as specialists in their subject area. Ether is added to the aqueous mixture.
In general aqueous or water-based solutions being polar are immiscible with non-polar organic solvents cooking oil chloroform toluene hexane etc and form a two-phase system. In the left separating funnel the aqueous layer is on the bottom meaning the organic layer must be less dense than water. 6 Compound Y has a distribution coefficient of 40 when extracted from water with chloroform with Y being more soluble in chloroform.
This can be done before lab by comparing densities but it can also be quickly determined in the lab. For example dissolving salt in. Without tasting smelling or removing either layer from the container how can you determine which layer is the aqueous layer using just material found in a typical kitchen.
Although the corner necessitates some clever cabinetry solutions to make it practical the open plan design of the L-shaped kitchen offers great flexibility in the placement of appliances and work zones. The separation is possible bc only the organic product is soluble in the organic solvent leaving inorganic impurities in the aqueous layer. Updated April 16 2021.

4 4 Which Layer Is Which Chemistry Libretexts
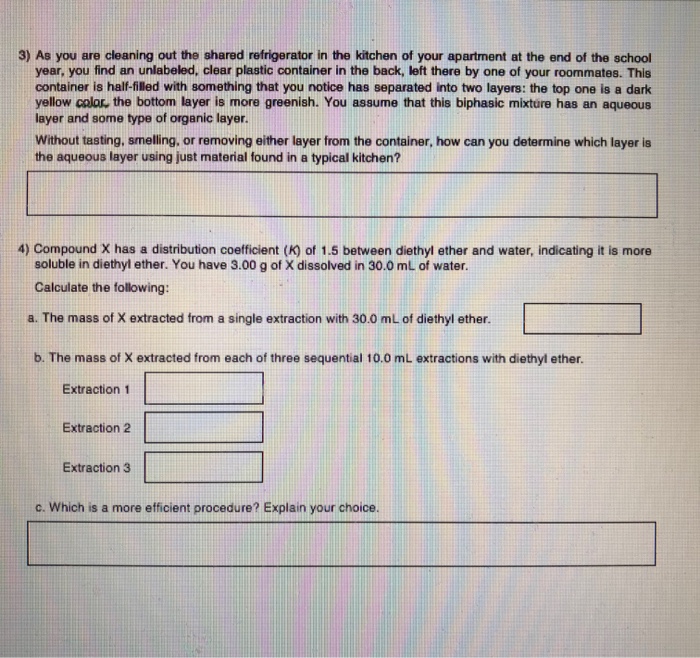
Solved 3 As You Are Cleaning Out The Shared Refrigerator In Chegg Com
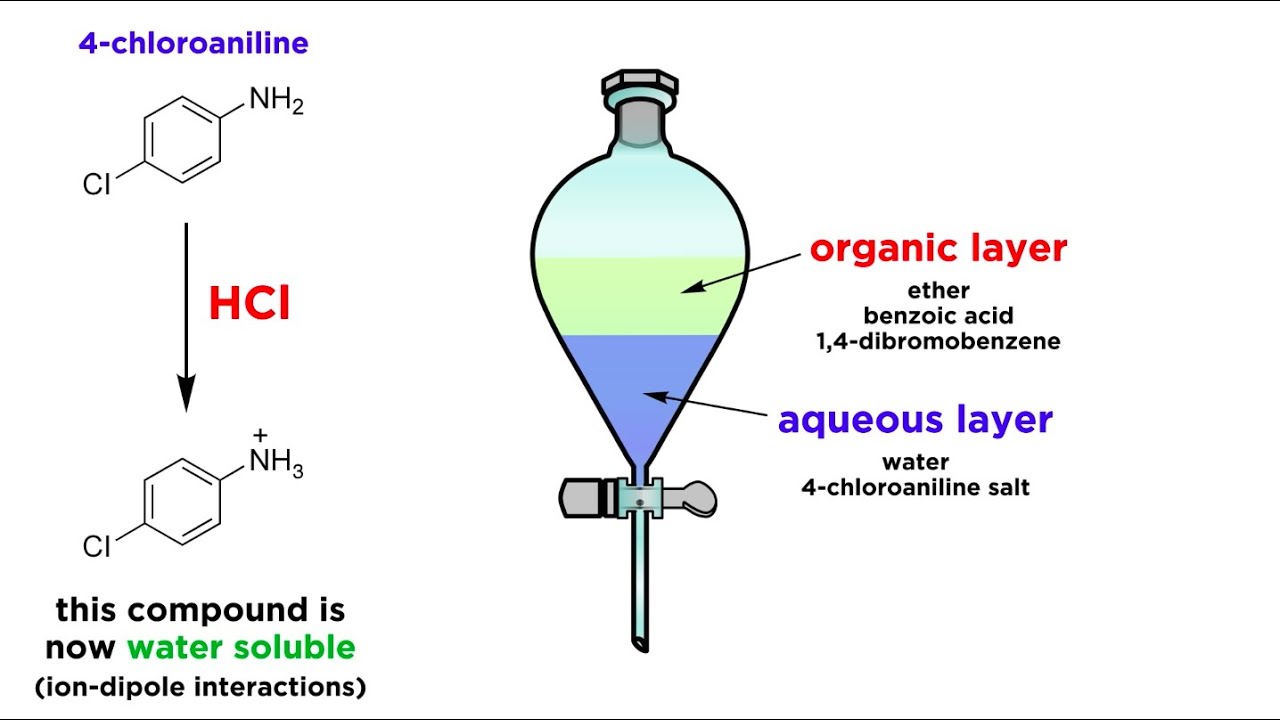
0 Response to "How to Determine Which Layer Is Aqueous Using Kitchen"
Post a Comment